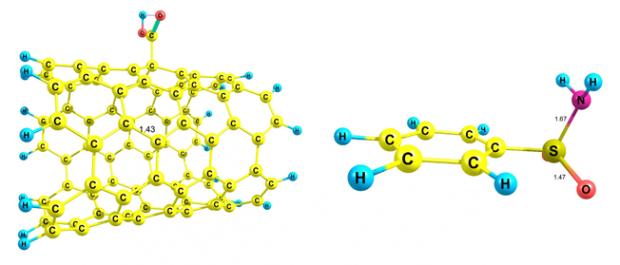
Mechanism of covalent adsorption of benzenesulfonamide onto COOH– and COCl– functionalised carbon nanotubes
Posted on 6. April, 2016.
The use of carbon nanotubes (CNTs) in drug delivery is a new, rapidly developing field. Different systems such as polymers, dendrimers, and liposomes have been used for drug delivery, but carbon nanotubes provide more effective structures due to their high drug loading capacities and good cell penetration qualities.
Although toxicity and low solubility create limitations in the applicability of CNTs, numerous reports concerning the use of CNTs as the carrier molecules for drugs have been presented. Most research in this field, at both theoretical and experimental levels, have been devoted to analysis of the possibility of the adsorption of drugs onto the sidewall of CNTs without considerable dysfunction in their electronic structures.
Using density functional theory, two mechanisms of covalent adsorption of benzenesulfonamide in water in the presence of functionalised carbon nanotubes were investigated. COOH– and COCl–functionalised carbon nanotubes can bond to the benzenesulfonamide via OH (OH pathway) and Cl (Cl pathway) groups, respectively. The activation energy and activation Gibbs free energy of the two pathways have been calculated and compared with each other. It was found that the OH pathway has an energy barrier higher than the Cl pathway and, in contrast to the Cl pathway, product formation is endothermic and non-spontaneous, being thus the reason for the dominance of the Cl pathway. All the calculations were performed using a hybrid density functional method (B3LYP) in the solution phase (polarised continuum model or PCM).
Read the full article in Progress in Reaction Kinetics and Mechanism, Volume 41, Number 1, 2016, pp. 100-107.
Authors: N.Darzi, A.Morsali* and S.A.Beyramabadi
Department of Chemistry, Mashhad Branch, Islamic Azad University, Mashhad, Iran
Keywords: functionalised carbon nanotubes, benzenesulfonamide, mechanism, activation energy, DFT, PCM
DOI:10.3184/146867816X14490560291507
Science Reviews 2000 now offers a personal subscription rate to non-institutional subscribers.
A 2016 online only, personal subscription to Progress in Reaction Kinetics and Mechanism is £60.00.
For more information or to subscribe, click here.